1.0. Introduction
A 64 years-old retired process worker, Mr. Black presented to GP’s surgery with an array of complaints. He had general aches in his joints mainly in wrists and ankles over the past few months. He had dyspnea and persistent cough which had developed over several months. He had assumed that this was due to ‘arthritis and advancing years’. Then he noticed that the tip of his fingers were becoming whitened and begun to feel cold. Also he stated that the skin on his finger and hands seemed to becoming tighter and disappearance of wrinkles. In the last few weeks his fingers on his left hand had started to become discolored at first and blackened.
He also complained of xeropthalmia and xerostomia along with dysphagia. He had become flatulent and had suffered from diarrhea followed by spells of constipation before the presentation at the surgery. Laboratory investigations were carried out in order to diagnose the possible underlying cause.
Table 1: Patient’s results obtained from the investigations (Labtestsonline, 2016, Gonzalez-Quintela, 2008)
| Investigation | Patient’s results |
|---|---|
| FBC | All parameters were within the normal range |
| ESR | 35 mm/hr – high (reference range: 12-20 mm/hr) |
| Serum electrophoresis | Marked, polyclonal, hypergammaglobulinaemia |
| Serum immunoglobulin levels | IgG = 35 g/l – high (reference range: 5.9-15.6 g/l) IgA = 8 g/l – high (reference range: 0.6-5.0 g/l) IgM = 12 g/l – high (reference range: 0.4-2.3 g/l) |
| X-ray | ‘Arthritic’ joints showed a thickening of the periarticular soft tissues and juxta-articular osteophorosis A grey mass was seen in the inferior lobe of the left lung |
| Urine test | Proteinuria |
| Skin biopsy analysis | Shows a thinning of epidermis with an accumulation of collagen within the reticular dermis. Arteriolar fibrosis was also evident. Also a lymphocytic infiltration was noted perivascularly (this was graded as mild). No further immunohistochemistry was done but these are assumed to be macrophages |
| Anti-nuclear antibody | Positive at a titre of 1/320. This antibody showed an anti-nucleolar pattern of staining on immunofluorescence |
| Anti-salivary duct antibody | Positive |
2.0. Discussion and diagnosis
2.1. Clinical suspicions/differential diagnosis related to the clinical presentation by the patient
The patient presented with an array of complaints indicating that his condition is systemic. He had general aches in his joints mainly in wrists and ankles over the past few months further indicative of a systemic condition. Considering his age, occupation and initial complaints, general aches in joints are mainly suggestive of rheumatoid arthritis (RA). Patients with RA may develop anaemia since the tissues in the inflamed joints may release proteins that affect the RBC synthesis by repressing the body’s ability to use iron causing rheumatoid anaemia. Thereby, reduces the RBC level (Masson, 2011). Based on the patient’s clinical presentation an FBC was performed to assess the patient’s general health and possible anaemic condition. FBC is usually a quantification of cellular components in the blood in order to check for the abnormalities in haematological parameters (Nabil, 2018). The patient’s results revealed normal for all the parameters indicating the absence of an infection or any anaemic condition. Therefore, suspicion of RA is excluded.
ESR was also performed and it is usually perform to assess possible underlying inflammation. ESR usually elevates in trauma, infection, cancers and in autoimmune diseases as (Inoue, 2018; Brigden and Malcolm, 1999). Patient’s ESR was 35 hr/mm indicating chronic inflammation which may be the reason behind his arthraglia.
He complained of dyspnea and persistent cough which had developed over several months. Mainly suggestive of a respiratory tract infection, lung cancer or autoimmune disease. SSc causes lung inflammation due to scarring and results in dyspnoea (Shiel, 2018). Dyspnoea occurs in SS as well (Ranatunga, 2018). However, progressive dyspnea and cough may be due to lung cancer or autoimmune disease since the possibility of infection was excluded with the aid of FBC. An X-ray imaging was performed based on the above symptoms to detect any abnormalities in body. In patient’s X-ray a grey mass was seen in the inferior lobe of the left lung further supporting the possibility of autoimmune disease and/or lung cancer (Hamatake et al., 2001). Patient’s with SSc is at a high risk of developing lung cancer (Avvedimento et al., 2013).
He had noticed that the tip of his fingers were becoming whitened and begun to feel cold. This process is also known as Raynaud’s phenomenon (RP). Raynaud’s phenomenon may occur as a result of vasoconstriction or ischemic condition in which there is an impairment of blood flow to the fingers causing low oxygenation. Low oxygenation may increase the muscle fatigue causing reduced muscle contraction lowering the release of heat energy making the fingers feel cold. Raynaud’s phenomenon can be commonly seen in SS, SSc and SLE (Shiel, 2018; Yoon, 2017; Viswanath, Phiske and Gopalani, 2013; Filipe et al., 2012). A Serum protein electrophoresis (SPE) was carried out to assess possible autoimmune condition. SPE is used to measure the specific protein levels in blood. The patient’s SPE showed marked, polyclonal hyper-gammglobulineamia. Polyclonal gammopathy is an indicative of autoimmune diseases and chronic infections mainly (Tuazon, 2014) (Figure 1).
But since the possibility of infection was ruled out its mainly suggestive of an autoimmune disease further supporting the possibility of SS, SLE and SSc.
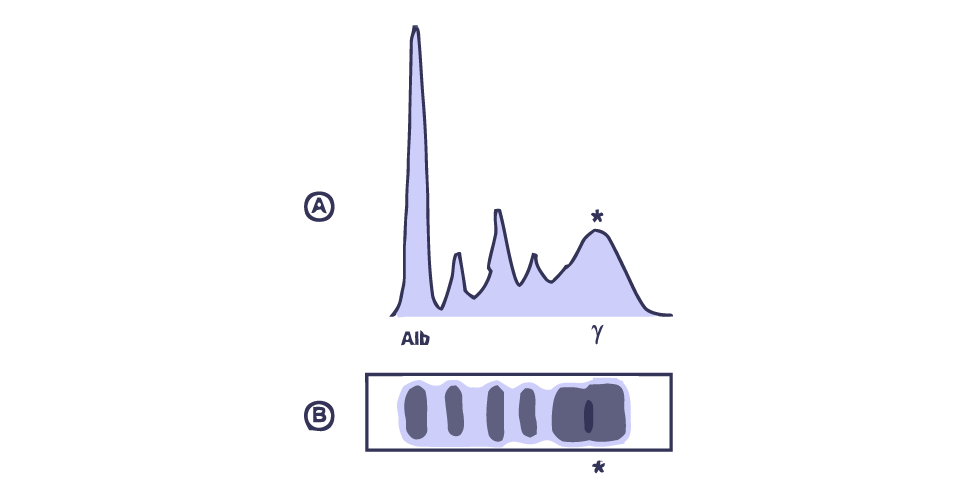
Figure 1: Polyclonal pattern serum SPEP
Also he stated that the skin on his finger and hands seemed to becoming tighter (sclerodactyly) and disappearance of wrinkles. Since the muscle contraction is less due to hypoxia, physiological alterations occur reducing the normal functioning of the tissues giving rise to tightened tissues (Giaccia, Johnson and Simon, 2004). Sclerodactyly occurs in patients with SSc, who are in indurative phase of SSc in which the skin becomes thick causing diminishing of the wrinkles. As well, in SLE and SS sclerodactyly occurs (Ardoin et al., 2017). This is due to deposition of collagen excessively. Fibroproliferation occurs under hypoxia giving rise to scar tissues causing impaired contraction, sclerodactyly and wrinkle disappearance (Cho et al., 2006). Patient’s X-ray further revealed the thickening of periarticular soft tissues and juxta-articular osteophorosis. These findings are further supportive of SSc and lupus arthritis manifested by SLE (Kunisz et al., 2014; Allanore et al., 2012; Ball and Bell, 2012).
In the last few weeks his fingers on his left hand had started to become discolored at first and then blackened. This may be due to reduced perfusion of finger tips giving rise to necrosis. Hypoxia may act as a mitogen and stimulate the melanocyte proliferation increasing pigmentation (Balin, Carter and Horikoshi, 1991). As well, during aging melanin production increases causing increased pigmentation (Budzisz et al., 2017). The patient’s image of left hand shows digital ulcers further suggesting SSc and SLE.
He also complained of xeropthalmia and xerostomia along with dysphagia. Due to low oxygenation mucus secretion from the salivary glands may reduce resulting in dysphagia. As well, xerostomia may occur as a result of oral dryness caused by salivary gland dysfunction. It can be caused by various systemic or autoimmune diseases such as SS, SSc and SLE (Bhattarai et al., 2018; Borges et al., 2015). Moreover, in SSc thickening of ocular tissues may cause xeropthalmia. As well, in SLE xeropthalmia occurs (Driver, 2018; Bartkowiak et al., 2013). Anti-salivary gland antibody test was done in which the presence of anti-salivary antibodies are detected via ELISA (He et al., 2015). The patient’s results were positive for anti-salivary antibodies showing the possibility of SS since this test is more specific for SS (He et al., 2015). However, in SSc also due to excessive fibrosis this test may show positive results and in SLE as well (Ranatunga, 2018).
In addition to other investigations, a serum immunoglobulin test which is a quantitative test that is done to assess the level of immunoglobulins was performed (Lab tests online, 2017). Patient’s results revealed elevation of IgG, IgA and IgM. Since there is no infection, elevation of immunoglobulins further supports the possibility of an autoimmune disesase (Castro and Gourley, 2010). IgG elevates in inflammation and play a role in anti-inflammatory response, IgA involves in anti-inflammatory responses in mucosal tissues showing an interconnection with patient’s symptoms such as xerostomia, xeropthalmia, dysphagia and constipation (Bos et al., 2007). Further supporting the possibility of SSc, SS and SLE (Bhattarai et al., 2018, Garcia-Carraso et al., 2013; Gupta et al., 2001).
He had become flatulent and had suffered from diarrhea followed by spells of constipation. This may be due to improper diet intake in patient due to dysphagia. Poor diet intake may increase the gastric acid secretion causing flatulence. Elevated ESR further supports the flatulence as a result of inflammation. Reduced mucus secretion may cause mal-absorption and hypoxia due to low perfusion increases gut microbiota to overgrow leading to diarrhea and flatulence as well (Marks, 2017; Creamy, Pierce and Peachey, 1969). As well low oxygenation reduces the mucus secretion from the gastric epithelium. Due to low mucus secretion low lubrication of faeces occurs and due to the reduced muscle contraction less functioning of the anal sphincter occurs causing difficulty in defecation leading to constipation (Hansson, Johansson and Sjovall, 2013). In SSc as a result of dysmotility which occurs due to fibrosis and myopathy, complications like diarrhea, constipation, overgrowth of bacterial flora occurs (Sheehan, 2008). In SLE, due to ischemia which occurs as a result of vasculitis or vascular occlusion and thickening of bowel leads to gastric complications such as diarrhoea (Cheong et al., 2001).
Considering all the symptoms and suspicions, Urine analysis, skin biopsy and anti-nuclear antibody test was also done to confirm the patient’s underlying disease. Urinalysis is a quantitative and qualitative analysis of urine (Echeverry, Hortin and Rai, 2010). Patient’s results revealed proteinuria. In SSc, renal arteriolar fibrosis causes the release of renin raising the serum renin levels leading to hypertension. This further activates the renin-angiotensin system angiotensin-II causing further vasoconstriction leading to renal ischemia. Thickening of renal vasculature and endothelial damage due to hypertension and ischemia may lead to renal crisis causing proteinuria (Hunzelmann et al., 2013; Hutchinson and Moots, 2001). Moreover, in autoimmune diseases auto-antibodies increases which may cause proteinuria since auto-antibodies are proteins. In SLE, auto-antibodies will deposit glomeruli and cause inflammation (Hornig, Mastroianni-Kirsztajn and Schlumberger, 2015).
Skin biopsy is perform to assess the pathological alterations in the tissues (Diaz and Weyers, 2002). Patient’s skin biopsy revealed a thinning of epidermis with an accumulation of collagen within the reticular dermis. Arteriolar fibrosis was also evident and lymphocytic infiltration was noted perivascularly. These findings reveal the reason behind the ischemia, tightening of the skin and wrinkle disappearance and fibrosis confirming systemic sclerosis and systemic scleroderma. Since SLE is not characterized by skin fibrosis its possibility is excluded (Schimeister et al., 2012; Jinnin, 2010).
Even though, anti-salivary antibody test which is specific for SS revealed a positive result suggesting the possibility of SS, skin fibrosis does not occur in SS and although SS co-exist as a secondary association with numerous rheumatic diseases such as SLE it is less likely to occur as a secondary association in patients with SSc. Hence the possibility of SS is excluded with the aid of skin biopsy (Ranatunga, 2018; Allanore et al., 2006).
Anti-nuclear antibody (ANA) test is commonly used to assess the possibility of an autoimmune disease. Patient’s ANA was positive at a titre of 1/320 indicating ANA positive further confirming the presence of autoimmune disease (Greidinger and Hoffman, 2003).
This antibody showed an anti-nucleolar pattern of staining on immunofluorescence. This pattern is more common in SSc further confirming SSc (Krzewska et al., 2008). Moreover, patient’s image of his left hand showed a lesion further confirming SSc (Kreig and Takehara, 2009).
Inclusion or exclusion of clinical suspicions were done with the aid of test results (Table 1).
Table 2: Exclusion/inclusion of differential diagnosis
| Suspected Condition/Disease | Symptoms/ investigations relating to the condition | Reasons for inclusion/ exclusion |
|---|---|---|
| RA | General aches and pains in joints, xeropthalmia, dysphagia, bowel habit changes, elevated ESR, hypergammaglobulinaemia | FBC is normal, Anti-salivary antibody test revealed positive results and the symptoms like flatulence is not present in RA. |
| Sjogren’s syndrome | General aches and pains in joints, xeropthalmia, xerostomia, dysphagia, gastric dysmotility, sclerodactyly, bowel habit changes, elevated ESR, hypergammaglobulinaemia, proteinuria, positive anti-salivary duct antibodies | Positive anti-salivary duct antibody test which is specific for SS |
| Systemic lupus erythematosus | General aches and pains in joints, Whitened finger tips and begun to feel cold, xeropthalmia, xerostomia, dysphagia, sclerodactyly, bowel habit changes, elevated ESR,arteriolar fibrosis, proteinuria, positive ANA | Skin biopsy revealed skin fibrosis and it does not support SLE. |
| Systemic scleroderma | General aches and pains in joints, Whitened finger tips and begun to feel cold, xeropthalmia, xerostomia, dysphagia, sclerodactyly, gastric dysmotility, bowel habit changes, flatulence, elevated ESR, hypergammaglobulinaemia, thickening of periarticular soft tissues and juxta articular osteophorosis, accumulation of collagen within dermis, arteriolar fibrosis, lymphocytic infiltration observed upon skin biopsy, proteinuria, positive ANA, anti-nucleolar pattern of ANA | Patient’s clinical symptoms and the test results obtained are strongly supportive of SSc. |
| Lung Cancer | Dyspnoea, persistent cough, presence of grey mass upon X-ray examination | X-ray findings supports the possibility of lung cancer. |
The results obtained from the investigations indicated possible systemic sclerosis and scleroderma complicated with lung cancer as the final diagnosis.
2.2. General immune mechanism of systemic scleroderma
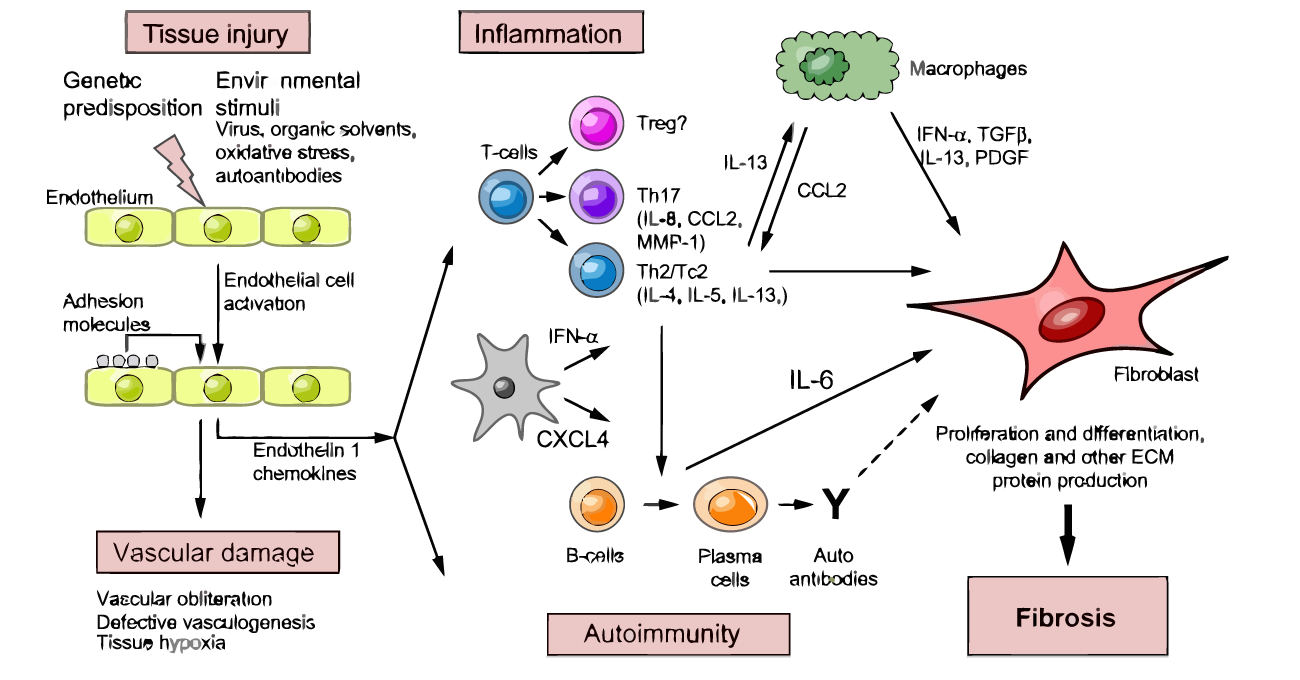
Figure 2: Pathogenesis of SSc
The main characteristics of SSc pathogenesis involves vasculopathy, altered humoral and cell-medaited autoimmunity, visceral and vascular fibrosis, endothelial damage and hypoxia. In exposure to oxidative stress, environmental stimuli, genetic predisposition and autoantibodies endothelial damage occurs. This causes up-regulation of adhesive, vasoconstrictive, mitogenic, thrombogenic factors and cytokines resulting in inflammatory cell infiltrations causing vascular leakiness leading to edema and inflammation. The infiltrates predominantly contain mononuclear CD4 T-cells and macrophages. Table 1 shows the cytokines/chemoattractants produced by macrophages, T-cells and its role in fibrosis. They mediate the thickening of tissues altering the normal functioning of the tissues. Whereas, fibrosis in vasculature causes narrowing of the lumen leading to hypoxia (Fuschiotti, 2016).
Table 3: The key cytokines involve in SSc fibrosis and their role (Adopted from Abraham et al., 2009)
| Cytokine | Role in Fibrogenesis |
|---|---|
| Tranforming growth factor-β (TGF-β) | ECM production, fibroblast proliferation and differentiation |
| CTGF | Regulation of fibroblast proliferation and migration and TGF-β-dependent ECM synthesis |
| Endothelin-1 (ET-1) | Regulation of ECM synthesis and contraction |
| Fibroblast growth factor | Regulation of fibroblast growth |
| IL-1 | Inflammatory mediator |
| IL-4 | Regulation of collagen synthesis |
| IL-6 | Regulation of α-SMA expression in myofibroblasts |
| IL-12 | Regulation of collagen synthesis |
| IL-13 | Induction of TGF-β |
| IL-17 | Fibroblast proliferation |
| Chemoattractant protein-1 (MCP-1) | Inflammatory mediators and regulation of collagen synthesis |
| MCP-3 | Regulation of collagen synthesis |
| Platelet derived growth factor (PDGF) | Regulation of TGF receptor expression, and fibroblast and progenitor cell recruitment |
T-cells divide into T-helper cells and T-regs usually. T-regs play a major role in maintaining self-tolerance and prohibit autoimmunity. However, a T-reg dysfunction occurs in SSc (Yamamoto, 2011). B-cells also get activated and produce auto-antibodies. Apart from that B-cells mediate T-cell activation and produce cytokines such as IL-6 and IL-10 which involves in collagen synthesis (Spertini and Zuber, 2006). Likewise, these mediators promote fibrosis in lungs, kidneys etc (Haase, 2012; Atmas and White, 2003).
2.3. Further investigations
Table 4 shows the further investigations to be carried out for a definite confirmation of diagnosis.
Table 4: Further tests that are needed to be carried out (Porter, 2017; Andolfi et al., 2016; Jimenez, 2016)
| Investigation | Reasons for the test |
|---|---|
| Serum CXCL4 | To assess the cardiovascular involvement in scleroderma and the degree of pulmonary fibrosis. |
| Bronchoscopy | Since the patient had dyspnea and persistent cough that progressed over several months and based on the X-ray finding of a grey cell mass in the inferior lobe of the lung suspicion of lung cancer aroused. Hence, a bronchoscopy can be done to reveal the reason behind the patient’s symptoms and X-ray results. The expected results would be the presence of a cell mass in inferior lobe of the lung. |
| Lung biopsy | A lung biopsy would be done to check if the cell mass is benign or malignant. |
3.0. Treatment plan
sNo cure is currently available for systemic scleroderma but the symptoms can be treated effectively with oral antibiotics which includes calcium-blockers, anti-inflammatory drugs, vaso-dialators etc. Hence, several treatment plans are suggested as shown below in table 5 in order to regain his quality of life.
Table 5: Prescribed drug regimen, surgical intervention and patient management (Schwartz, 2018; Klabunde, 2017; Ogbru, 2016; Patel and Sahen, 2014; Cutolo et al., 2001; Nicholas, Reidy and Steen, 1992)
| Scleroderma-related complication | Recommended treatment | Mechanism of action |
|---|---|---|
| Vasculopathy | Nefedipine | Reduces peripheral resistance and act as a vaso-dialator by blocking Ca2+ channels in smooth muscle fibers and relaxes the vascular smooth muscles inhibiting vaso-constriction. |
| Inflammation of joints | NSAIDS like ibuprofen | Reduces inflammation |
| Skin fibrosis | Methotrixate | Reduces the monocytic cell growth and increases their apoptosis, decreases IL-1 & IL-6 which contributes in fibroproliferation, decreases Th1 cytokines which are IL-2 & IFNγ synthesis reducing pro-inflammation. Induces apoptosis of activated T-cells as well. |
| Lung fibrosis | Cyclophosphamide | Act as a immuno-suppressive agent suppressing T-cell & B-cell activity & production |
| Raynaud’s phenomenon | Bosentan | Act as an endothelin receptor antagonist inhibiting ET-1 which directly activate fibroblasts to make collagen, reverse the vasospasm |
| GIT | Omeprazole/Histamin-2 | These are proton pump inhibitors which reduces the acid reflux, inflammation and flatulence |
| Renal crisis | ACE/angiotensin II inhibitor | Causes vaso-dialation and reduces blood pressure |
| Xerostomia and Xeropthalmia | Moisturizing eye drops for xeropthalmia Pilocarpine/Cevimelin for xerostomia | Prevent the dryness in the eyes and mouth |
| Surgical intervention | Amputation | Amputation should be carried for the surgical removal of necrotic tissues of his left (digital ulcers) hand. |
| Patient management | Dietary management | Patients oesophagial dysmotility should avoid hard solid food intake and increased intake of high fiber diets |
| Patient management | Activity management | Maintenance of patient’s core body temperature to avoid raynaud’s phenomenon Avoid contamination of skin lesions Digital ulcers should be kept clean and dry Instruct the patient to engage in physical activities to avoid join contractures |
4.0. Conclusion
Since the patient’s symptoms were linked with many systems of the body such as respiratory, GI, integumentary and renal system the condition was suspected to be systemic. The results indicated and symptoms indicated possible systemic sclerosis and scleroderma complicated with lung cancer as the underlying cause for the patient’s symptoms and patient management and precise treatments are recommended.
5.0. Abbreviations
ANA Anti-nuclear antibody
CTGF Connective tissue growth factor
ESR Erythrocyte sedimentation rate
ET-1 Endothelin-1
FBC Full blood count
MCP-1 Chemoattractant protein-1
PDGF Platelet derived growth factor
RA Rheumatoid arthritis
SLE Systemic lupus erythematosus
SPEP Serum protein electrophoresis
SS Sjogren’s syndrome
SSc Systemic scleroderma
TGF-β Tranforming growth factor-β
6.0. References
Abraham, D.J., Disler, J., Distler, O. and Kreig, T. (2009) ‘Overview of pathogenesis of systemic sclerosis’, Rheumatology, 48(3), pp.33-37.
Allanore, Y., Ardizonne, M., Avocue, J., Depiny, C., Khan, A., Silbilia, J., Sordet, C., Vacher-Lavaneu, M.C. (2006) ‘Systemic sclerosis-associated Sjogren’s syndrome and relationship to the limited cutaneous subtype: Results of a prospective study of sicca syndrome in 133 consecutive patients’, Arthritis & Rheumatism, 54(7).
Allanore, Y., Avouac, J., Clements, P.J., Furst, D.E. and Khanna, D. (2012) ‘Articular involvement in systemic sclerosis’, Rheumatology, 51(8), pp.1347-1356.
Andolifi, M., Capozzi, R., Liparulo, V., Potenza, R., Puma, F. and Yasufuku, K. (2016) ‘The role of bronchoscopy in the diagnosis of early lung cancer; a review’, Journal of Thoracic Disease, 8(11), pp.3329-3337.
Ardoin, S.P., Meter, H.V., Reed, A.M., Robinson, A.B. and Torok, K.S. (2017) ‘Pediatric systemic lupus erythematosus, juvenile dermatomyositis scleroderma and vasculitis’, in Budd, R.C., Firestein, G.S., Gabriel, S.E., Mclnnes, L.B., O’Dell, J.R. (ed.) Kelly and firestein’s Textbook of Rheumatology, 2, pp.1844-1875.
Atmas, S.P. and White, B. (2003) ‘Cytokine regulation of pulmonary fibrosis in scleroderma’, Cytokine & Growth Factor Reviews, 14(6), pp.537-550.
Avvedimento., E.V., Bonifazi, M., Gabrielli, A., Gabrielli, B., Negri, E., Pomponio, G., Tramacere, I. and Vecchia, C. (2013) ‘Systemic sclerosis (scleroderma) and cancer risk: systemic review and meta-analysis of observational studies’, Rheumatology, 52(1), pp.143-154.
Baker, H. and Golding, D.N. (1968) ‘Primary raynaud’s disease associated with sclerodactyly and digital osteosclerosis’, Postgraduate Medical Journal, 44(513), pp. 553-555.
Balin, A.K., Carter, M. and Horikoshi, T. (1991) ‘Effects of oxygen tension on the growth and pigmentation of normal human melanocytes’, The Journal of Investigative Dermatology, 96(6), pp.841-844.
Ball, E.M.A and Bell, A. (2012) ‘Lupus arthritis-do we have a clinically useful classification’, Rheumatology, 5(1), pp.771-779.
Bartkowiak, B.D., Gos, R., Jurowski, P., Waszczkowska, A. and Waszczkowska, E. (2013) ‘Prevalence of ocular manifestations in systemic sclerosis patients’, Archives of Medical Science, 9(6), pp.1107-1113.
Benzing, T., Hunzelmann, N., Kreig, M., Kurschat, C., Moinzadeh, P. and Schuster, J. (2013) ‘Proteinuria in systemic sclerosis: reversal by ACE inhibition’, Rheumatology International, 33(9), 2225-2230.
Bhattarai, K.J., Chae, H., Handigund, M., Junjappa, R. and Kim, H. (2018) ‘the imprint of salivary secretion in autoimmune disorders and related pathological conditions’, Autoimmune Reviews, 17(4), pp.376-390.
Borges, A.H., Dias, E.P., Espinosa, M.M., Fernandes, V., Galera, M.F., Leite, C.A. and Lima, P.R.T. (2015) ‘Prevalence of hyposalivation in patients with systemic lupus erythematosus in a Brazilian subpopulation’, International Journal of Rheumatology, 2015, pp.1-6.
Brigden, M.D. and Malcolm, L. (1999) ‘Clinical utility of the erythrocyte sedimentation rate’, American Family Physician, 60(5), pp.1443-1450.
Budzisz, E., Rotsztejn, H., Skoczynska, A. and Trznadel-Grodzka, E. (2017) ‘Melanin and lipofusin as hallmarks of skin aging’, Advances in Dermatology and Allergology, 34(2), pp.97-103.
Castro, C. and Gourley, M. (2010) ‘Diagnostic testing and interpretation of tests for autoimmunity’, Journal of Allergy and Clinical Immunology, 125(2), pp. 238-247.
Cheong, W.K., Chua, K.B., Luman, W. and Ng, H.S. (2001) ‘Gastrointestinal manifestation of systemic lupus erythemosus’, Singapore Medical Journal, 42(8), pp.380-384.
Cho, C.S., Choi, J.J., Hong, K.H., Kang, S.S., Kim, W.U. and Yoo, S.A. (2006) ‘Hypoxia induces expression of connective tissue growth factor in scleroderma skin fibroblasts’, Clinical & Experimental Immunology, 146(2).
Cutolo, M., Pizzorni, C., Seriolo, B., Straub, R.H. and Sulli, A. (2001) ‘Anti-inflammatory mechanisms of methotrexate in rheumatoid arthritis’, Annals of Rheumatic Diseases, 60(8).
Diaz, C. and Weyers, W. (2002) ‘Basic principles of skin biopsy’, Pathology, 23(1), pp.4-8.
Echeverry, G., Hortin, G.L. and Rai, A.J. (2010) ‘Introduction to urinalysis: historical perspective and clinical application’, The Urinary Proteome, 641, pp.1-2.
Filipe, P., Freitas, J.P. Gomes, M.M., Miguel, D., Pinheiro, C. and Uva, L. (2012) ‘Cutaneous manifestations of systemic lupus erythematosus’, Autoimmune Diseases, pp.1-15.
Fuschiotti, P. (2016) ‘Current perspectives on the immunopathogenesis of systemic sclerosis’, Immuno Targets and Therapy, 5, pp.21-35.
Garcia-Carraso, M., Jimenez-Hernandez, C., Jimenez-Hernandez, M., Mendoza-Pinto, C., Nava-Zavala, A. and Reibeling, C. (2012) ‘Serologic features of primary Sjogren’s syndrome: clinical and prognostic correlation’, International Journal of Clinical Rheumatology, 7(6), pp.651-659.
Giaccia, A.J., Johnson, R. and Simon, C.M. (2004) ‘The biology of hypoxia: the role of oxygen sensing in development, normal function and disease’, Genes & Development, 18(18), pp.2183-2194.
Gonzales-Quintela, A., Alende, R., Gude, F., Campose, J., Rey, J., Meijide, L.M., Fernandez-Merino, C. and Vidal, C. (2008) ‘Serum levels of immunoglobulins (IgG, IgA, IgM) in a general adult population and their relationship with alcohol consumption, smoking and common metabolic abnormalities’, Clinical & Experimental Immunology, 151, pp.42-50.
Greidinger, E.L. and Hoffman, R.W. (2003) ‘Antinuclear antibody testing: Methods, indications and interpretations’, Laboratory Medicine, 34(2), pp.113-117.
Gupta, V., Pande, S.S., Seth, M., Singh, R.G. and Suri, A. (2001) ‘Immunological alterations in scleroderma’, Journal, Indian Academy of Clinical Medicine, 2(3), pp.185-188.
Haase, V.H. (2012) ‘Hypoxia-inducible factor signaling in the development of kidney fibrosis’, Fibrogenesis and Tissue repair, 5(1).
Hamatake, M., Ishida, T., Sugimachi, K. and Yamazaki, K. (2001) ‘Lung cancer with p53 expression and a solitary metastasis to the stomach: a case report’, Annals of thoracic and cardiovascular surgery, 7(3), pp.162-165.
Hansson, G.C., Johansson, M.E.V. and Sjovall, H. (2013) ‘The gastrointestinal mucus system in health and disease’, Nature Reviews: Gastrointerology and Hepatology, 10(6), pp.352-361.
He, J., Hua, H., Li, C., Li, Z., Qiang, L. and Wei, P. (2015) ‘Role of salivary anti-SSA/B antibodies for diagnosing primary Sjogren’s syndrome’, Medicina Oral, Patologia Oral y Cirugia Bucal, 20(2), pp.156-160.
Hornig, N., Mastroianni-Kirsztajn, G. and Schlumberger, W. (2015) ‘Autoantibodies in renal diseases-Clinical significance and recent developments in serological detection’, Frontiers in Immunology, 6(221), pp.1-6.
Hutchinson, D. and Moots, R.J. (2001) ‘Late onset Raynaud’s phenomenon, hypertension, deteriorating renal function, and a fit in a middle aged woman’, Postgraduate Medical Journal, 77(10).
Inoue, S. (2018) Medscape. Available at: whttps://emedicine.medscape.com/article/956278-overview#5 (Accessed: 06 June 2018).
Jimenez, S. A. (2016) Scleroderma Workup. Available at: https://emedicine.medscape.com/article/331864-workup#c6 (Accessed: 30 July 2018).
Jinnin, M. (2010) ‘Mechanisms of skin fibrosis in systemic sclerosis’, The Journal of Dermatology, 37, pp.11-25.
Klabunde, R.E. (2017) Cardiovascular Pharmacology Concepts. Available at: https://cvpharmacology.com/vasodialator/ACE (Accessed: 28 July 2018).
Kreig, T. and Takehara, K. (2009) ‘Skin disease: a cardinal feature of systemic sclerosis’, Rheumatology, 48(3), pp.314-318.
Krzewska, I., Palaz, A., Pyka, J. and Zabek, J. (2008) ‘Antinucleolar antibodies in diagnostics of antiphospholipid syndrome’, Polish Archives of Internal Medicine, 118, 25-30.
Kunisz, W., Matuszewska, G., Saied, F., Smorawinska, P., Sudol-Szopinska, I., Wloddkoswska-Korytkowska, M. and Zaniewicz-Kaniewska, K. (2014) ‘Radiological imaging in pediatric rheumatic diseases’, Polish Journal of Radiology, 79, pp.51-58.
Lab tests online (2017) AACC. Available at: https://labtestsonline.org/tests/quantitative-immunoglobulins (Accessed: 21 July 2018).
Labtestsonline.org. (2016) Erythrocyte Sedimentation Rate (ESR). [Online]. Available at: https://labtestonline.org/tests/erythrocyte-sedimentaionrate-esr (Accessed: 27 June 2018).
Marks, J.W. (2017) Medicine Net. Available at: https://www.medicinenet.com.intestinal_gas_belching_bloating_flatulence/article.htm (Accessed: 30 July 2018).
Masson, C. (2011) ‘Rheumatoid anaemia’, Joint, Bone Spine, 78, pp.131-137.
Nabil, S.N. (2018) Medicine Net. Available at: https://www.medicinenet.com/complete_blood_count/article.htm# (Accessed: 19 July 2018).
Nicholas, J.J., Reidy, M.E., and Steen, V. (1992) ‘Lower extremity amputation in scleroderma’, Archives of Physical Medicine and Rehabilitation, 73(9), pp.8811-813.
Ogbru, O. (2016) Medicine Net. Available at: https://www.medicinenet.com/nifedipine/article.htm (Accessed: 28 July 2018).
Patel, R. and Sahen, A. (2014) ‘The epidemiology of Sjogren’s syndrome’, Journal of Clinical Epidemiology, 6, pp.247-255.
Porter, A. (2017) Medical News Today. Available at: https://www.medicalnewstoday.com/articles/317874.php (Accessed: 28 July 2018).
Ranatunga, S.K. (2018) Medscape. Available at: https://emedicine.medscape.com/article/332125-clinical (Accessed: 26 July 2018).
Schmeister, T., Pons-Kuhnemann, J., Ozden, F., Muller-Ladner, U. and Dinser, R. (2012) ‘Arthritis in patients with systemic sclerosis’, European Journal of international Medicine, 23, pp.25-29.
Sheehan N.J. (2008) ‘Dysphagia and other manifestations of oesophageal involvement in the musculoskeletal diseases’, Rheumatology, 47(6), pp.746-752.
Shiel, W.C. (2018) MedicineNet. Available at: https://www.medicinenet.com/raynauds_phenomenon/article.htm (Accessed: 25 July 2018).
Spertini, F. and Zuber, J.P. (2006) ‘Immunological basis of systemic sclerosis’, Rheumatology, 45(1), pp.323-325.
Tuazon, S.A. (2014) Medscape. Available at: https://emedicine.medscape.com/article/2087113-overview#a1 (Accessed: 26 July 2018).
Viswanath, V., Phiske, M.M. and Gopalani, V.V. (2013) ‘Systemic sclerosis: Current concepts in pathogenesis and therapeutic aspects of dermatological manifestations’, Indian Journal of Dermatology, 58(4), pp.255-268.
Yamamoto, T. (2011) ‘Autoimmune mechanisms of scleroderma and a role of oxidative stress’, Self Nonself, 2(1), pp.4-10.
Yoon, J.C. (2017) Medscape. Available at: https://emedicine.medscpe.com/article/1064663-clinical#b2 (Accessed: 25 July 2018).




