
Introduction to apoptosis and mechanism of apoptosis
The process of programmed cell death is known as apoptosis. It is done by defined morphological attributes and biochemical processes which depend on energy. It is the permanent termination of all the metabolic activities of the cell which is important in genetically determined cell elimination. Cell death is based on the activation of death genes and suicide pathway enzymes (Damjanov, 2009). There is a signaling pathway of controlling, determining the cell cycle and cell apoptosis. Cell shrinkage, blebbing, alteration of cell membranes, nuclear fragmentation, and chromatin condensation are common features of apoptosis (Elmore, 2007).
Apoptosis plays a vital role during physiological processes of fetal development and in adult tissues. Errors of apoptosis cause disorders such as cell accumulation like cancer, restenosis, and cell lose issues such as stroke, heart failure, neurodegeneration, AIDS (Kalinichenko and Matveeva, 2008). There are mainly 2 pathways of apoptosis as Extrinsic and intrinsic (Figure 1). Intrinsic pathway initiates due to the intracellular signals produced by cells which are stressed and depends on the proteins produced in mitochondrial intermembranous space. The extrinsic pathway is activated by extracellular signals and the cell suicide itself causing the formation of the death-inducing signaling complex. Extrinsic pathway or the death receptor pathway begins when death ligands bind to death receptors. This consist mainly TNF1 receptors and a protein Fas. Their related ligands are TNF and Fas ligands respectively. Both the pathways are induced by caspases. Caspases are a protease in more specifically activated form of intracellular cysteine proteases family which divides its substitute in the presence of aspartic acid residues. Caspases mean C for cysteine, ASP for aspartyl-specific and ases for proteases. out of many pathways in activating caspases intrinsic and extrinsic pathways are talked in detail. There are two types of caspases are engage in apoptosis, initiator caspase, and effector caspase. Initiator caspases are caspase-2,-8,-9,-10 and caspase-3,-6,-7 are effector caspase (Elmore, 2007).
In addition to these two pathways, another pathway that involves T cell-mediated cytotoxicity and perforin-granzyme-dependent killing of the cell occurs. Perforin/granzyme pathway occurs via either granzyme A or B (Elmore, 2007).
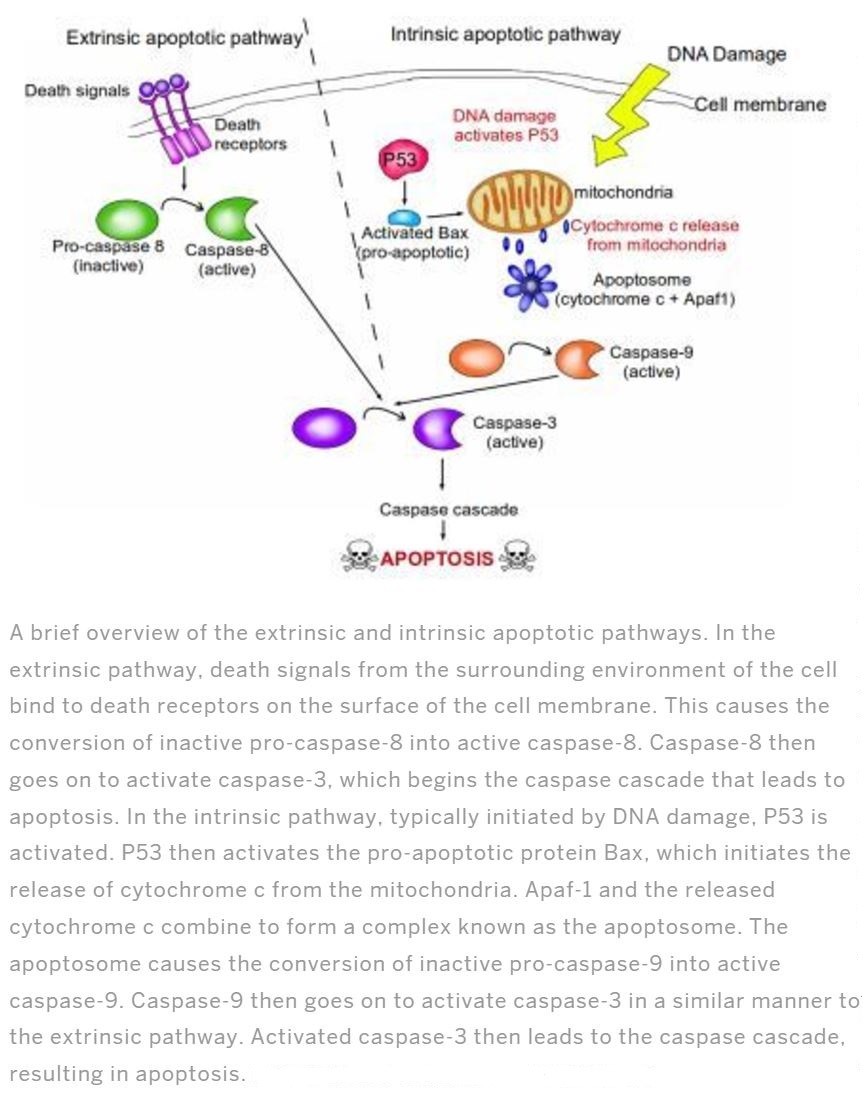
These pathways converge on same terminal or the execution pathway and cause the cleavage of caspase-3 (Figure 2).
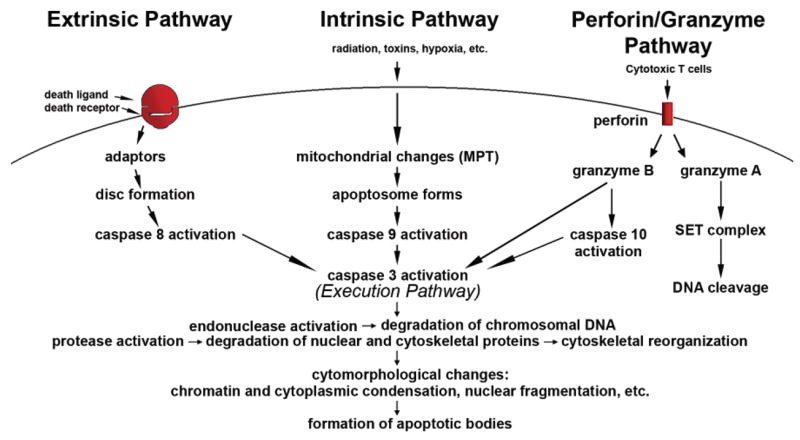
This results in DNA fragmentation, degradation of cytoskeletal and nuclear proteins, the formation of apoptotic bodies and expression of ligands for phagocytic cells thereby uptake by the phagocytic cells (Figure 3). The importance of apoptosis is, it takes place within the development and cell aging and helps to maintain the homeostasis by controlling the number of cells in tissues. This mechanism is useful when there are damaged cells due to diseases, infection, injury or carcinogenic agents. Similar to apoptosis there is another mechanism knowingly necrosis. This releases the cell content by rupturing the cell membrane. Stimulus determines whether the cell dies by necrosis or apoptosis. High dose of harmful stimuli may induce necrosis whereas low dose may induce apoptosis (Elmore, 2007).
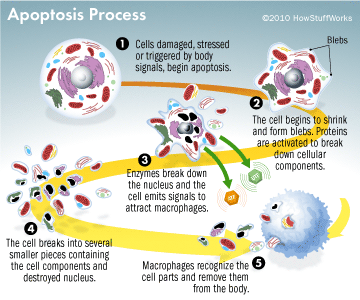
How normal cells become abnormal and the reduction of apoptosis
When the cells of the multicellular organisms function correspond to the rules of growth and reproduction the only organism can grow. But there are sometimes where cells break the rules where cells divide rashly affecting the other cells sometimes causing the death to the organism. Abnormal cells like cancer cells disrupt the functions of the normal cells. These abnormalities are causes of inherited mutations or are triggered by environmental factors like UV light, X-rays, chemicals and tobacco products. In situations like cancers, cancer cells weaken the control systems which prevent the overgrowth of cells and invasion of other tissues by the cancer cells. And in the presence of signals normally hinder the cell growth the altered cells or cancer cells grow and divide. Due to this signals which trigger the cell growth and division is no longer required. With the growth of these cells, new cell characteristics are fostered such as alterations in cell structure, reduced cell adhesion, and production of new enzymes. These abnormal cells spread and invade the other tissues even in the presence of normal cells which obstruct the growth of cells. Mutations occurred in protein-encoding genes which regulate the cell division causes abnormalities in cancer cells. With the time more genes get mutated leading to more and more deformities in the mother cells and the daughter cells. Ultimately this leads to malignant cells that will not die due to the too little apoptosis or the apoptosis resistance (Lowe and Lin, 2000).
Oncogenic mutations disturb apoptosis causing tumor initiation and metastasis. And some oncogenic alterations induce apoptosis (Lowe and Lin, 2000). Apoptosis is known as the method which monitors the elimination of excess and damaged cells. Apoptosis and cell cycle is regulated by no.of key genes, proteins, and enzymes. Regulators of apoptosis belong to Bcl2 family. Bcl2, Bcl-XL, Bcl-W, Mcl-1, and A1 are some examples of anti-apoptotic proteins that inhibit apoptosis while Bax, Bad, Bid, Bok, Bik, and Bak are pro-apoptotic proteins which induce apoptosis. The p53 is an extremely important protein in apoptosis mechanism which is produced by TP53 and its regulatory protein along with the RB1 gene and its related protein pRB inhibit the cell proliferation. Mutations occurred in these key genes during cancer development affects the regulation of proteins and enzymes which cause disruptions for the cell proliferation (Samarasinghe, 2013).
The resistance of apoptosis may be due to distrupted balance of pro-apoptotic and anti-apoptotic proteins of Bcl-2 family, reduced caspase function, Inhibition of apoptosis proteins and impaired death receptor signaling (Figure 4).
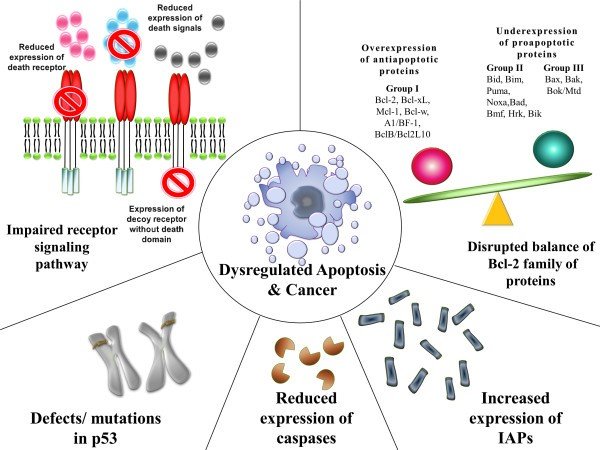
In the disruption of the balance of pro-apoptotic and anti-apoptotic proteins; ratio of pro and anti-apoptotic proteins play a vital role during the cell death regulation where they reported due to their activity on the cell. In cells over and underexpression of some genes such as the resultant regulatory proteins take part in carcinogenesis by cutting down apoptosis in cancer cells. In situations where imbalance created between anti and pro-apoptotic proteins of the Bcl-2 family (pro and apoptotic proteins form the Bcl-2 family of proteins which play a vital role in the apoptosis regulation.) apoptosis in the malignant cells become dysregulated (Wong,2011; Alberts, 2002).
Inhibitors of apoptosis proteins (IAP’s) are structurally and functionally alike proteins that involve the regulation of apoptosis, signal transduction, and cytokinesis. Dysregulation of these proteins is one of the causes of many cancers. Another way is reduced caspase activity. Caspase play vital role in apoptosis. Since there are two types the caspases 1 involves mainly in cytokine mechanisms in mediating inflammatory responses and caspase 2 involves in apoptosis. As mentioned previously caspase 2 divides as initiator and effector caspases. They initiate and execute apoptosis. Decrement of caspase cause reduction in apoptotic process and stimulate carcinogenesis (Wong,2011).
Impaired death receptor signaling is another way of evading apoptosis by malignant cells. In the extrinsic pathway of apoptosis is related to death receptors and ligands of them. Irregularities in death signaling directly affect the extrinsic pathway of apoptosis where evasion apoptosis occurs (Wong,2011).
Induction of apoptosis in the cancerous cells and treatments
Since apoptosis helps to maintain the genetic stability as well the defective apoptosis can lead to carcinogenesis or tumorigenesis. Suppression of apoptosis promotes the cancer progression. Tumor cells suppressed apoptosis can be achieved by molecular mechanisms such as the expression of anti-apoptotic proteins like Bcl-2, down-regulation of pro-apoptotic proteins like BAX, targeting Cell Cycle Control System, targeting DNA Repair System for Apoptosis Induction and targeting IAP-family members XIAP, cIAP1, Survivin, or Apollon can induce apoptosis (AbuAlmaaty, 2014).
Cancer treatments target mainly apoptotic inhibitors including Bcl-2 family proteins, IAPs, and c-FLIP for apoptosis induction. By targeting anti-apoptotic Bcl-2 family members, in here there are 3 strategies in an attempt to overcome the cytoprotective effects of Bcl-2 and Bcl-xL attacking the proteins directly with small molecule drugs, shutting off gene transcription and inducing m-RNA degeneration with antisense oligonucleotides trigger the activity of apoptosis genes. Other than anti-sense oligodeoxynucleotides apoptosis can be induced by some another drugs which aim on Bcl-2. And Mcl-1 is known for the induction of apoptosis in malignant cells. And the second strategy is targeting cell cycle control system for apoptosis induction. Under several types of stress conditions p53 triggers cell cycle arrest and apoptosis to regulate genetic integrity and prevent the passage of damaged DNA to the daughter cells (Figure 5) (AbuAlmaaty et al, 2014; Bucur, Khosravi and Plati, 2011; Ashkenazi and Herbst, 2008).
Induction of apoptosis is targeted on the abnormal cells over normal cells. This can be stimulated by pro-apoptotic pathways too. If this pathway is stimulated cancer cells will show more sensitivity towards death (Ashkenazi and Herbst, 2008).
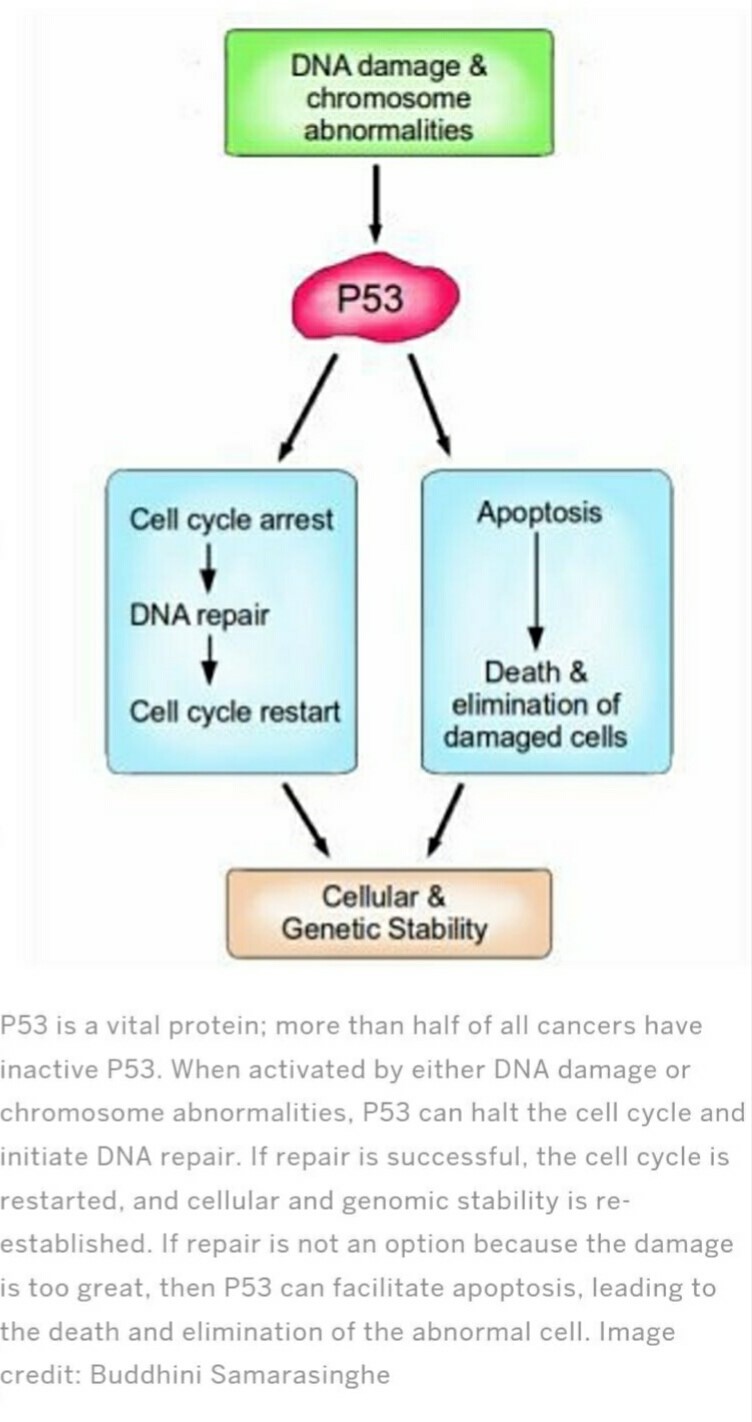
Cancer treatments such as radiation and chemotherapy induce apoptosis with its morphological features. Drugs, radiation therapies, and chemotherapy can cause DNA damage in some cells which may lead to induce apoptosis via p53 dependent pathway (Elmore, 2007). And also anticancer agents trigger apoptosis which proves that drug interaction has an effect on drug-induced cell death. As well re-introduction of the typical p53 gene to mutant form can trigger apoptosis and tumor regression. But p53 is not the only one which induces apoptosis, all anticancer agents with the sufficient dosage can trigger programmed cell death. And it is independent from p53. Anticancer agents induce apoptosis in normal cells and tumors. Therapy-induced apoptosis can be triggered with the help of death receptor pathways (Lowe and Lin, 2000). Some of the therapeutic drugs that are given to induce apoptosis are 5-Fluorouracil (5-FU), Antimicrotubule Agents (AMA) and Cisplatin. Sometimes cancerous cells will overcome the apoptosis induced by this therapeutics (Hassan et al, 2014). As well the drugs such as steroid dexamethasone directly trigger apoptosis in malignant cells (Gerl and Vaux, 2005).
Ultrasound is not only used in the examination but also in therapy for different illnesses including cancers. During cancer therapy using the ultrasound, those waves penetrate through the cells and tissues under certain conditions and alter the permeability of cell membrane directly and it can induce the apoptosis within the cells in both in vitro and in vivo conditions. It is found that low frequency and low-intensity ultrasound waves trigger apoptosis which is enhanced with the contribution of sonodynamic sensitivities, microbubbles, chemotherapeutic drugs and more (Bai, Hu, and Shen, 2012).
It is very clear that the process of programmed cell death or apoptosis is an important mechanism to maintain the equilibrium in the body’s cells. The main pathways of cell apoptosis are Intrinsic, extrinsic and granzyme pathway. Suppression of normal cell suicide pathways is a major contributory factor for the progression of cancer. Suppression can be occurred due to impaired receptor signaling, reduced expression of caspases, increased expression of IAP’s, disrupted the balance of Bcl-2 family of proteins and mutations in the p53. Therefore it is necessary to overcome the mechanisms contribute to the evasion of apoptosis and induce apoptosis in these cells to eliminate the proliferation of cells unnecessarily.
Cancer treatments and therapies focus on the induction of apoptosis both in-vitro and in-vivo. Sometimes cancer cells may overcome the programmed cell death induced by the therapeutics and the cancer treatments. However, rapid progress in the apoptosis may reduce the risk of cancer progression.
References
- AbuAlmaaty, A., Hassan, M ., Ohba, Y., Sakuragi, N. and Watari, H., (2014) ‘Apoptosis and Molecular Targeting Therapy in Cancer’, Biomed Research International, 2014(2014), pp.23. Hindawi [Online] DOI: http://dx.doi.org/10.1155/2014/150845 (Accessed: 28 May 2017).
- Alberts, B., Johnson, A., Lewis, J., Raff, M., Roberts, K. and Walter, P. (2002) Molecular Biology of the Cell. 4th edn. New York: Garland Science.
- Ashkenazi, A. and Herbst, R.S. (2008) ‘To kill a tumor cell: the potential of proapototic receptor agonists’, The Journal of Clinical Investigation, 118(6), pp.1979-1990. JCI [Online] DOI: 10.1172/JCI34359 (Accessed: 01 June 2017).
- Bai, W., Hu, B. and Shen, E. (2012) ‘The induction of the apoptosis of cancer cell by sonodynamic therapy’, Chinese Journal of Cancer Resaerch, 24(4), pp.368–373. PMC [Online] DOI: 10.3978/j.issn.1000-9604.2012.08.03 (Accessed: 28 May 2017).
- Bucur, O., Khosravi-Far, R. and Plati, J. (2011) ‘Apototic cell signaling in cancer progression and therapy’,Intergrative Biology, 3(4), pp.279-296. Royal Society of Chemistry [Online] DOI: 10.1039/C0IBOO144A (Accessed: 01 June 2017).
- Damjanov, I. (2009) Pathology Secrets. 3rd edn. Mosby Elsevier.
- Elmore, S. (2007) ‘Apoptosis: A Review of Programmed Cell Death’, Toxicologic Pathology, 35(4), pp.495-516.
- PMC [Online] DOI: 10.1080/01926230701320337 (Accessed: 27 May 2017).
- Elmore, S. (2007) ‘Apoptosis: A Review of Programmed Cell Death’, Toxicologic Pathology, 35(4), pp.495-516.
- SAGE journals [Online] DOI: 10.1080/01926230701320337 (Accessed: 27 May 2017).
- Gerl, R. and Vaux, D.L (2005) ‘Apoptosis in the development and treatment of cancer’, Carcinogenesis, 26(2), pp.263-270. Oxford Academic [Online] DOI: https://doi.org/10.1093/carcin/bgh283 (Accessed: 28 May 2017).
- Kalinichenko, S.G. and Matveeva, N.Y. (2008) ‘Morphological characteristics of apoptosis and its significance in neurogenesis’, Neuroscience Behavior Physiology, 38(4), pp.333-344. PMC [Online] DOI: 10.1007/s11055-008-0046-7 (Accessed: 29 May 2017).
- Lowe, S.W and Lin, A.W. (2000) ‘Apoptosis in cancer’, Carcinogenesis, 21(3), pp.485-495. Oxford Academic [Online] DOI: https://doi.org/10.1093/carcin/21.3.4 (Accessed: 27 May 2017).
- Samarasinghe, B. (2013) Scientific American. Available at: https://blogs.scientificamerican.com/guest-blog/hallmarks-of-cancer-3-evading-apoptosis/ (Accessed: 29 May 2017).
- Wong, R.S.Y. (2011) ‘Apoptosis in cancer: from pathogenesis to treatment’, Journal of Experimental & Clinical Cancer Research, 30(1), pp.87. PMC [Online] DOI: 10.1186/1756-9966-30-87 (Accessed: 27 May 2017).




